04 Apr EPCIS Terms to Know
In helping you on your EPCIS journey, we provide a list of commonly used terms you will hear as serialization progresses over the next few months. Of course, some may already be familiar to you as you have heard them since DSCSA was enacted.
Data Carrier—A GS1 term for the different kinds of media, such as barcodes, that can hold GS1 identification keys and application identifiers.
Downstream—The direction in which product flows in a supply chain. Generally speaking, pharmaceutical products flow, and transactions occur through the supply chain from manufacturers, repackagers, wholesale distributors, and dispensers.
DQSA—The Drug Quality and Security Act. (DQSA) U.S. Federal legislation passed in November 2013. DSCSA is a section of DQSA.
DSCSA—The Drug Supply Chain Security Act (DSCSA) is Title II of DQSA. DSCSA mandates an entire supply chain traceability system from pharmaceutical manufacturer to pharmacy dispenser for prescription drugs distributed in the United States. The law was signed by President Obama in November 2013, providing a national standard for drug security and replacing the patchwork of state-level Pedigree regulations that were in place.
EDI—Electronic Data Interchange. The electronic transfer of data between computer systems in a standardized message format.
EDI 856—Advance Ship Notice (ASN), the common name for the EDI 856 transaction. EDI 856 is a notification of pending deliveries in an electronic format.
EPCIS—Electronic Product Code Information Services. A GS1 EPCglobal standard designed to enable EPC-related data-sharing within and across enterprises. This data-sharing aims to allow participants in the EPCglobal Network to obtain a standard view of the disposition of EPC-bearing objects within a business context. (More at www.gs1.org/epcis).
External Product Identifier—A standards-based product code, such as a Global Trade Item Number (GTIN) or a market-specific product code, used to identify the product in the external supply chain. An external product identifier is not a manufacturer SKU, which is not regulated or standardized.
GCP—Global Company Prefix. A globally unique code used to represent a location in identifiers. See also GS1 Company Prefix.
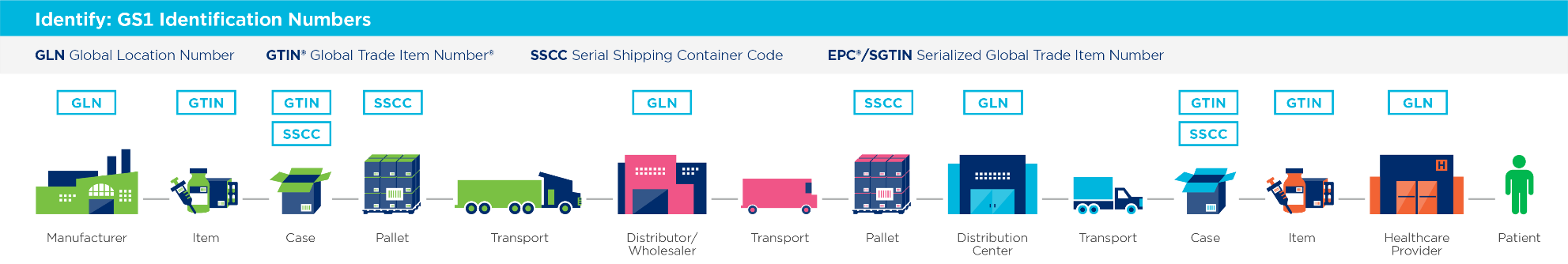
GLN—Global Location Number. A unique 13-digit number containing a GS1 company prefix, a location reference, and a check digit is used to uniquely identify a physical location or legal entity in the supply chain. The GLN makes possible the unique and unambiguous identification of those locations and entities.
Global Identifier—A unique reference number used to identify a legal entity such as a company or location, to support the secure exchange of business information on the Internet.
Grandfathering—A provision in which some pre-existing situations are not subject to new rules or regulations.
GS1—A leading global organization dedicated to designing and implementing global standards and solutions to improve the efficiency and visibility of supply and demand chains globally and across sectors. The GS1 system of standards is the world’s most widely used supply chain standards system. (More at www.gs1.com).
GS1-128—A linear barcode, formerly referred to as a Code-128 barcode. Usage is granted to organization members of GS1.
GS1 Company Prefix—A globally unique identifier for a company, assigned and administered by GS1 Global. The GS1 Company Prefix is 4 to 12 digits and is a component of GLN, GTIN, and SSCC identifiers.
GS1 Data-matrix—A two-dimensional matrix barcode consisting of black and white “cells” or modules arranged in a square or rectangular pattern. The information to be encoded can be text or raw data. Usage is granted to organization members of GS1.
GTIN—Global Trade Item Number. An identifier for trade items developed by GS1. These identifiers are used to look up product information in a database, often by inputting the number via a barcode scanner pointed at an actual product. The uniqueness and universality of the identifier help establish which product in one database corresponds to which product in another, especially across organizational boundaries. Usage is granted to organization members of GS1.
HDA (formerly HDMA)—Healthcare Distribution Alliance (formerly the Healthcare Distribution Management Association). The national association in the U.S. represents primary, full-service healthcare distributors. HDA member companies deliver more than nine million prescription medicines and healthcare products to more than 165,000 settings, including chain and community pharmacies, hospitals, nursing homes, physician offices, and clinics in every state and territory.
Interoperability—The ability of technology systems and software to communicate, exchange data and/or information, and use the information being exchanged.
Master Data—Data representing a company’s details, global identifiers, products, and trading partners. Particular types of data are required for serialization and global compliance reporting.
Serial Number—Typically a portion or component of a Unique Identifier (UID) which provides uniqueness. Also known as a serial reference.
SGLN—Serialized Global Location Number. A unique identifier to a physical location, such as a specific building or bin within a warehouse. The GLN is a GS1 format; the SGLN is an EPC format and is represented in Uniform Resource Identifier format, for example, urn:epc:id:sgln:123456 789012..0.
SGTIN—Serialized Global Trade Item Number. The combination of a Global Trade Identification Number (GTIN) and a serial number uniquely identify an item.
SSCC—Serial Shipping Container Code. A GS1 standard used in logistic encoding and communications. The SSCC ensures that logistic units are identified with a number that is unique worldwide.
T3—Under DSCSA, the combination of Transaction Information (TI), Transaction History (TH), and the Transaction Statement (TS) for a drug product as it moves through the drug supply chain.
TH—Transaction History. A record of transaction information for each change of ownership within the supply chain, starting with the manufacturer.
THS—Transaction Histories. Documentation for transaction exchanges of multiple products, including (for each product) all three transaction documentation components: TI, TH, and TS.
TI—Transaction Information. A comprehensive set of details about each product included in a transaction, including product name, National Drug Code (NDC) number, strength and dosage form, size and the number of containers, lot number, date of transaction, and the names of the companies involved in the transaction.
Transaction—As defined by DSCSA, the transfer of product where a change of ownership occurs. Exemptions: intercompany distributions, distribution among hospitals under common control, public health emergencies, dispensed pursuant to a prescription, product sample distribution, blood, and blood components for transfusion, minimal quantities by a licensed pharmacy to a licensed practitioner, charitable organizations, distributions pursuant to a merger or sale, certain combination products, certain medical kits, certain IV products, medical gas distribution, and approved animal drugs.
TS—Transaction Statement. A statement confirming that trading partners are authorized by law to transfer ownership of product, have received transaction documentation, have systems in place to comply with verification requirements, and did not knowingly ship suspect product or provide false information.
Traceability—The process of tracking drugs through the supply chain using serialization data. Track and trace systems begin with serialization but generally include additional components such as product tracing or tracking, verification, and/or reporting.
UID—Unique Identifier. A string of numbers and characters that is unique within a given system. Examples include GS1, GTIN, and GS1 SSCC identifiers.
UPC—Universal Product Code. The U.S. standard article number. A form of GTIN data carrier or barcode.
Upstream—The opposite direction that product normally flows in a supply chain, moving back up the supply chain. Generally speaking, the pharmaceutical product flows, and transactions occur through the supply chain from manufacturers, repackagers, wholesaler distributors, and dispensers.
VAN—Value Added Network. A hosted service offering that acts as an intermediary between business partners sharing standards-based or proprietary data via shared business processes.
Trademark Notices
DataBar®, EAN®, EPC®, EPCglobal®, GDSN®, GS1 Global Registry®, GTIN®, and Global Trade Item Number®are registered trademarks of GS1 AISBL.
GS1 US®and design is a registered trademark of GS1 US, Inc. Trademarks appearing in this presentation are owned by GS1 US, Inc. unless otherwise noted, and may not be used without the permission of GS1 US, Inc.
The letters “U.P.C.” are used solely as an abbreviation for the “Universal Product Code” which is a product identification system. They do not refer to the UPC, which is a federally registered certification mark of the International Association of Plumbing and Mechanical Officials (IAPMO) to certify compliance with a Uniform Plumbing Code as authorized by IAPMO.
Nasarenko, T. (2023). GS1 Identification Numbers [Photograph]. Healthcare Distribution Alliance (HDA).